Wednesday, 30 November 2016
The Universe in 101 words: Where is night permanent?
Earth spins at a 25 degree tilt, but the Moon’s tilt is almost zero*.
So Earth’s poles get 6 months of day then 6 months of night… but at the Moon’s poles the Sun never rises or sets, just moves around the horizon. A deep hole on the Lunar pole (like a crater or valley), so deep you couldn’t see the Sun over the rim, would be cut off from it.
Permanently.
Billions of years of asteroids have created many polar craters like that. Some haven’t seen the Sun for billions of years, and have strange things frozen in them...
*Incidentally this is why we get seasons, and the Moon doesn’t.
Monday, 28 November 2016
Answers for Authors: Where do planet forming elements come from?
 |
| Above: A picture. Worth a thousand words? I don't know, but it's a galaxy, so it could be home to a lot of space aliens |
They say a picture is worth a thousand words.
I’d argue that a picture of say, a horse, is worth much less than a thousand words of accurate advice on undetectably rigging the lottery.
But that’s just me being contrary, as the picture we need to look at is actually a map(s) of our galaxy:
True, it looks like a Frisbee made of glowing mist, but that's actually over two hundred billion stars (one of which is our Sun), each with a complex relationship to all the others. We can simplify it down to three bits however:
- The central bulge - very densely packed stars.
- The disc/arms - sparser stars, of which our Sun is one.
- The wispy halo - very sparse stars, but with occasional dense clusters.
The bright clusters in the halo are things called globular clusters: Huge, round, families of old stars often separated by less than the width of our solar system. The central bulge also has a lot of old stars, and everything is very close packed, but with some very active areas of new star growth. The disk is mostly made of newer stars.
Where’re the heavier, planet forming, elements concentrated on our map?
Mostly in the central bulge, amongst the super jam packed stars there. There’s a fair bit scattered throughout the disk, but very little in the halo of globular clusters.
Why?
That’s down to how elements heavier than helium were made: Our Universe formed (we think) with a lot of hydrogen, a fair bit of helium, and a tiny trace of heavier atoms – not nearly enough to form lots of planets, and our galaxy has around a hundred billion of those.
The heavier atoms were mostly formed by hydrogen and helium atoms smashing into each other and fusing together – something that only happens under very hot and dense conditions, like a star’s core. The fusing together of these light elements to produce heavier ones gives off energy, which is how the star shines. Eventually it dies – which usually mean it blows off its outer layers - and the heavy atoms it’s forged get spread through the local dust an gas clouds. Those eventually form new stars, with the heavier elements mixed into them.
It’s the circle of life! Except much bigger, and with stars instead of lions.
 |
| "So, Mufasa, will you be eating me at the end of the song or do I make it to the second act? I am holding you're son, sire." |
And no singing warthogs, because no one needs singing warthogs on that scale.
Where it can, the process repeats – producing stars and nebula clouds with even more heavy elements, and eventually the concentrations can get high enough for rocky planets like Earth to form. If we look at it like that, it’s obvious the heavier elements will be found where there have been more generations of stars - and our galaxy started birthing stars in the central bulge, about 13.5 billion years ago.
 |
| Pwooooaaaaarrrr, check out that central bulge! |
Then there was a mysterious pause, and then the stars began forming in the disk about 5 billion years later. That’s a pause longer than the age of Earth, so astronomers clearly still have some work to do here, but the point is:
- The galactic bulge is the oldest part of our galaxy where star birth is still going on, so there’s been lots of time for heavy elements to build up there – typically 2-3 times the concentration of heavy elements found in our Sun.
- While the disk hasn’t seen as many generations of star growth, it’s seen enough for a reasonable amount of heavy elements to build up – planets are common enough, stars generally have 0.5 to 1 times the Sun’s metal content.
- The wispy halo of globular clusters is the most heavy element poor bit of the galaxy – they’re incredibly ancient places**, but they haven’t seen much in the way of new star growth during their entire lifespan, so there’s not much built up there.
They're actually ash from the most violent events in the universe: Supernova.
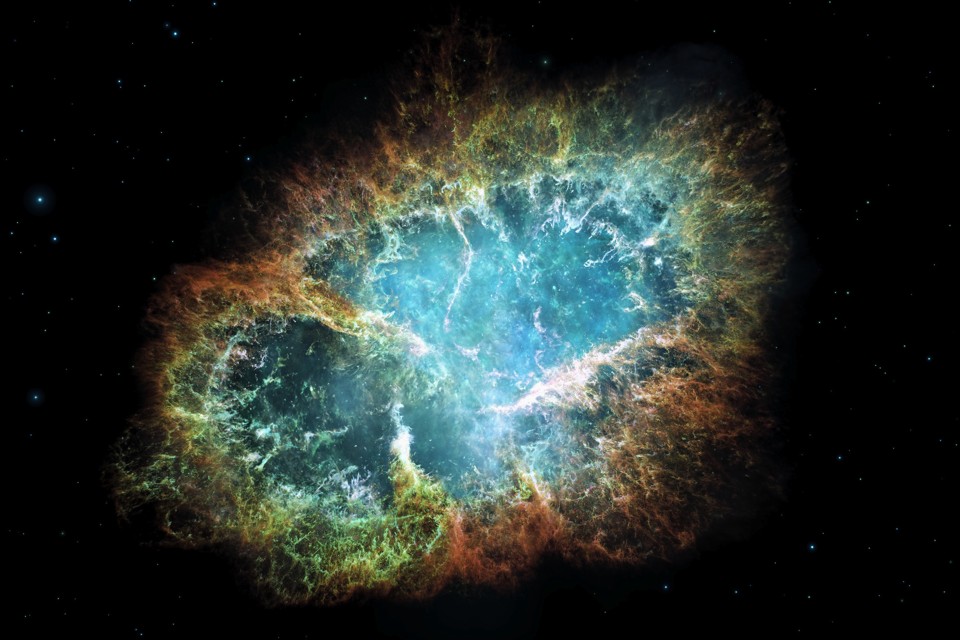 |
| Above: The Crab Nebula, a huge debris cloud from a supernova. |
Only the biggest and hottest stars can even get their fusing as far as iron. Big, super hot, stars only lead relatively short lives of a few hundreds of millions of years or less – so unlike most stars they never travel very far from the nebula where they’re born. When they die the supernova floods the inert iron 'ash' in the core with neutrons, which combine with the nuclei of the iron atoms to make heavier elements.
For that reason supernova sites, like the Crab Nebula above, are hugely enriched in radioactive elements (heavy atomic nuclei are much more likely to be unstable), and the gas clouds around the supernova all get a hefty dose too.
Normals sized, long lived, stars and their solar systems form from those clouds, and drift away from the heavy element forming hotbeds. Over time their radioactive elements decay - that’s why our solar system originally had a lot more radioactivity – enough for things like the naturally occurring Oklo nuclear reactor to run 2 billion years ago.
So that’s the general outline: Our galaxy has a heavy element rich middle bulge (which is a mix of very ancient metal poor stars and very metal rich young stars), a disk moderately well supplied with heavier elements, and a halo globular clusters made of very heavy-element-poor ancient stars. Scattered throughout both the core and the disk are star forming regions, which are the source of the unstable, radioactive, super heavy elements.
If you’re looking for more specific locations where huge concentrations of super-heavy elements could be found though, here are some suggestions:
Neutron star planets:
Whole worlds can be born from death on a cosmic scale - or, to put it a bit less melodramatically, from the remains of a supernova: When a central remnant, usually a neutron star, is formed it can hold a swirling disk of debris from the old stars core around itself. That disk can then undergo the same kind of transformation that the protoplanetary disks around young stars undergo, and produce planets. Massively rich in radioactive elements, and bathed in high energy radiation they're orbiting the superdense corpse of a dead star. They rejoice in names like Draugr, Poltergeist and Phobetor - because astronomers love an opportunity to dredge up the names of old, dark entities and put them to use.
 |
| Above: A map of a neutron star solar system. |
These planets wouldn’t be black as night though: As well as the constant volcanic eruptions caused by radioactive heat, the neutron star itself would retain the heat from the supernova for a long time, illuminating them as an incredibly intense pinprick of very slowly fading light over millions of years.
Neutron star collision sites:
It’s not unknown for two supergiant stars to form as a binary system, then die and leave behind two orbiting neutron stars. Over time those neutron stars lose their orbital energy, and spiral in towards each other. The two city sized objects, each so dense that a pinhead of their matter would weigh more than a supertanker, spin around each other at nearly the speed of light, until they merge together. A neutron star can be thought of as a 20km wide atomic nucleus and the debris from two giant atomic nuclei colliding at nearly lightspeed is, unsurprisingly, incredibly rich in heavy elements. In fact if you’re wearing a piece of gold right now the odds are good that it was made in one of these insanely overpowered events.
Above: A simulation of colliding neutron stars, courtesy of NASA. Because what else is NASA going to do with a super computer?
Personally I'd love get a chance to learn more about these places... although, given the levels of radiation, hard vacuum, incredible heat, and general death... probably not by visiting.
* It’s not great place to look for aliens, because the jam packed stars make a stable planetary system hard to form, and the constant barrage of radiation makes it hard for complex life to develop, but it’s sure got a lot of heavy elements.
**We’re not clear on why, or why they never made any new stars, but at least that’s keeping astronomers from roaming the streets in packs.
Wednesday, 23 November 2016
The Universe in 101 words: What is the EM drive?
 |
| Above: An ElectroMagnetic drive. |
So far, so not exciting or strange. But fill it with microwaves, at a frequency that resonates within the box, and it feels a tiny pull.
H
That's weird: As far as anyone can tell, nothing is leaving the box, nothing is pushing on the box.... it shouldn't feel any force. Yet some NASA tests suggest this effect might be real.
Better experiments might reveal a mundane explanation. But, if that tiny force is real, a whole new kind of physics may be waiting to be discovered...
Monday, 21 November 2016
The Universe in 101 words: Did the Moon ever have an atmosphere?
Yes.
Today’s Lunar atmosphere is real, but sparse: It’s only a trillionth the pressure of Earth’s. Very occasionally it's added to by underground reservoirs, created by decaying radioactive elements.
But, although the Moon's low gravity loses gas fast, it was once a volcanic world: For a billion years the gases spewed out by vast chains of volcanoes (and occasional giant impacts) gave the Moon an atmosphere occasionally as thick as Mars’.
That’s thick enough for wind, clouds, frosts – a dynamic world. Once, if you looked up at the Moon, you’d have seen lunar clouds blowing across it’s face…
Wednesday, 16 November 2016
6 strangely beautiful videos of water in space:
 |
| Above: Saturn's ocean moon, Enceladus. |
If you're talking about weightlessness, on a space station or space ship, it becomes a very strange and beautiful thing:
Water balloons in microgravity don't burst, they just reveal strange morphing shapes:
Water doesn't run anywhere in microgravity, so wash-cloths don't work:
And if you add an effervescent tablet to coloured water you can make... this:
But large enough amounts would only freeze at the surface. Which mean's there are world's covered in water and ice, like Jupiter's moon Europa where a 100km deep ocean huddles below kilometres of ice:
You've read our science facts - have you tried our science fiction? You can pick up our book on Kindle, or as a paperback, by following this link...
"Like Phillip K Dick tweeting" - Tageblatt magazine
"Like Phillip K Dick tweeting" - Tageblatt magazine
...or, if you'd like to help us do things like podcasts, animations, and videos here why not...
Monday, 14 November 2016
Answers for Authors: Where can your characters get antimatter from?
Learn the ins and outs of pseudoscience, and debunking it: Read 'Escaping The Rabbit Hole' here.
Want to support the future science and engineering? You can do so through one of these charities:
Skeptoid media
The Association for Science Education
The Smallpiece Trust
SATRO
Arkwright Engineering Scholarships
LabAid
The Space Foundation
Anyway... the short answer is: Saturn's rings.
 |
| There you go. Cup of tea? |
Ah, you'd like details? Well:
Antimatter is often called normal matter's 'opposite', 'mirror image', or 'dark twin'. Which is very dramatic, and makes physicists feel cool saying it, but doesn't explain much.
I'm a physicist, but I've made peace with my cool quotient being pretty damn low. I think of it like this: If a particle of matter can be defined by a set of numbers - it has an electric charge of 2 for example - then it's antimatter equivalent would have the same numbers but with a '-' in front*. So an electric charge of 2 for a matter particle becomes an electric charge of -2 for its antimatter equivalent, and so on. The interesting bit, from a spaceflight (or killing things) perspective, is that when the particle and antiparticle meet they disappear in a flash of energy.
And you can be sure that's true, because no sane person would make something like that up and expect you to believe it.
So, if you mix antimatter with regular matter you can make astounding amounts of energy: A gram of antimatter, if released into our environment, would explode with the force of a medium sized nuke.
Brilliant! Where do I get some**?
 |
| Come on, you don't seriously think an incredible new energy source wouldn't be weaponised before breakfast? |
You won't be surprised to hear that a material that explodes on contact with anyything isn't just lying around. But it's not just rare: For reasons we genuinely don't understand, our universe is made of matter only - there just isn't any natural antimatter.
We know antimatter can exist, because we can make tiny amounts of it with particle accelerators. Our best theories of the Universe's beginning predict that it should have begun wit equal quantities of each. But, somehow, everything has ended up made of matter - which is a huge mystery, and another reason scientists would love to get their hands on enough to investigate better.
But how? It can only be found inside the most powerful particle smashers, in incredibly minute quantities? We could set up huge atom smashers, optimised for antimatter production, all over the place. But, at a cost of 65 trillion dollars a gram, mass production might be a bit impractical.
 |
| This is $1 trillion - the speck on the lower left is a person. I can't recall if the stack is of twenties or fifties, but it's a lot to spend for a gram of something. |
Is there another way?
Cosmic rays – fast moving particles hailing from who know’s-where-in-deep-space – form antimatter when they collide with planetary ring systems and atmospheres. The reaction is roughly analogous to reactions that produce antimatter in particle accelerators, but a planet's atmosphere or ring system is hugely bigger, and 'runs' non stop for billions of years. As the antiparticles produced are electrically charged they can get stuck in the planets magnetic field, whizzing around it until they hit some stray bit of matter and go bang.
Even though the amounts produced are much better than current accelerators, they are still minute – there are 160 nanograms over Earth, and even Saturn’s mighty rings only produce a quarter of a milligram a year.
 |
| Sorry Scotty. |
But, used cleverly, even that is enough to power a super fast spaceship. The trick is that antimatter doesn’t have to be used as a fuel in itself, it can be used as a catalyst to make engines using less powerful fuels work better - like giving a car nitrous.
Although not much like, unless nitrous does vapourise the car and 'Fast and The Furious' just left that bit out.
Both nuclear fission and nuclear fusion reactions could be made much easier to start and keep running with a pinch of antimatter thrown into the mix - which makes building a nuclear powered engine a lot easier. And a spaceship powered by an antimatter catalysed engine could reach Mars in three months, for example.
Antimatter doesn't just go bang - for example a teensy dose of antimatter delivered to the right spot could be used to kill cancer cells. And it could us why our universe is a safe place made of matter, not an constantly exploding matter/antimatter cauldron.
Since it might be so useful, there are plans to extract it: James Bickford put forward a plan for a constellation of satellites to collect antimatter from Earth’s upper atmosphere, and store it in strong magnetic fields. It could be collected, and returned to Earth as needed.
If we could get those same satellites out to Saturn we could collect enough antimatter to spark a dozen revolutions in particle physics, medicine, and space ship propulsion - although we’d need a revolution in spaceship propulsion first:
We'd need fleets of satellites needed to cover the massive acreage of satirn’s ring s and collect significant amounts.
But, if you were rocking an antimatter catalysed fusion engine in the year 2223, you might well find yourself refuelling over Satirn’s rings. Which means that, unlike at most petrol station forecourts, you’d get one of the best views in the solar system as you filled you tank.
* Not quite all - for example inertial mass and gravitational attraction are the same
** Like most physicists, I am 2% supervilian. It's part of the initiation... I've already said to much...
Wednesday, 9 November 2016
The Universe in 101 words: Is your house on an impact site?

Yes, it is.
How do I know?
Earth’s over four billion years old - plenty of time for huge asteroid strikes. But four and a half billion years ago Earth got hit, not by an asteroid… but by another planet.
‘Bang’ doesn’t cover it: The explosion completely melted Earth, leaving it a huge flying blob of lava surrounded by a ring of molten debris.
That's effectively day zero for Earth – anything older was obliterated. The debris ring became the Moon* – a very different Moon than it is today.
And you're standing on the aftermath, right now...
* Or possibly Moons.
You've read our science facts - have you tried our science fiction? You can pick up our book on Kindle, or as a paperback, by following this link...
"Like Phillip K Dick tweeting" - Tageblatt magazine
"Like Phillip K Dick tweeting" - Tageblatt magazine
...or, if you'd like to help us do things like podcasts, animations, and videos here why not...
Monday, 7 November 2016
Six of the strangest and most revealing space images
 |
| Credit: M. Kaufman; B. Saxton (NRAO/AUI/NSF); ALMA (ESO/NAOJ/NRAO); NASA/ESA Hubble Space Telescope. |
The Cosmic Horseshoe:
 |
| Credit: ESA/Hubble & NASA |
OK, there are hundreds (it's space after all), but we're only interested in the orange blob-like one and the blue arc-like image of the one behind it: The orange one is a gigantic elliptical galaxy, and it's huge gravity is warping the space around it. That space warp acts like a titanic lens, magnifying the blue galaxy in the background.
Because a lens of warped space doesn't work quite like a glass lens the image is distorted into a ring, but it still lets astronomers study the blue galaxy in far greater detail than they otherwise could
Schiaparelli crash site:
 |
| Credit: NASA/JPL-Caltech/Univ. of Arizona |
NASA's Mars Reconnaissance Orbiter is investigating the crash site, where Europe's Schiaparelli test lander hit the surface last week. More details here.
Pillars of destruction:
 |
| Credit: ESO/A. McLeod |
The pillars were imaged with the MUSE instrument on ESO's Very Large Telescope, by a team led by Anna McLeod, a PhD student at ESO. The name comes from the pillar's fate: The stars that shaped them will eventually blow them apart.
NASA and U.S. Navy practice recovering Orion:
Orion is NASA's new super space capsule, designed to carry astronauts to destinations including an asteroid and Mars.
Here, in a joint operation with NASA, the US navy are rehearsing the recovery of an Orion capsule after splashdown.
China's heavy lift rocket takes to the skies:
China's powerful new heavy-lift rocket lifted off from the Wenchang launch centre on Hainan Island, off China's southern coast, at 8:43 a.m. EDT November 3rd, according to Chinese media reports. China is (I think) weirdly secretive about it's space launches, but word is the payload carried to orbit was an experimental satellite called Shijian-17, which is designed to test electric-propulsion technology.
Curiosity Rover studies fallen iron meteorite:
The dark, smooth-surfaced object at the center of this picture from NASA's Curiosity rover is a iron-nickel meteorite - the same kind that regularly falls on Earth - sitting on the Martian desert.
The grid of shiny points visible on the object resulted from that laser zapping by Curiosity's Chemistry and Camera (ChemCam) instrument - it shoots stuff with a laser and studies the gasses emitted to see what it's made of.
I swear it wasn't there to start with.
Well. I couldn't be totally sure of that without being on Mars.
I'm reasonably sure - find out more about it here.
Wednesday, 2 November 2016
The Universe In 101 Words : Why is water on Mars so important?

An artists impression of a pool of hypersaline water on Mars.
Because everyone wants to find ET.
Where is there's water, on Earth, there's life.
On Mars the temperature, air pressure, and soil chemistry conspire to make an environment on the tipping point, where liquid water goes from being just possible to truly impossible.
But our robots have found that things there were more friendly to water billions of years ago - ancient Mars was much more inviting.
So Mars could let us test an important idea: Does water really always equal life?
Subscribe to:
Comments (Atom)














